Best Indicator For Hcl And Naoh Titration
This is because the pH range of Methyl orange and Phenolphthalein. Methyl orange would not be appropriate here as the color change occurs at between 31 and 44 in an aqueous solution.

Titration Chemistry For Non Majors
Therefore titrating one against the other yields a complete reaction.

Best indicator for hcl and naoh titration. We place 10000 ml of the HCl solution in a flask with a drop of an indicator that will change color when the solution is no longer acidic. In the discussion above we decided that we could use bromothymol blue or phenol red as indicators for the titration of NaOH aq a strong base with HCl aq a strong acid because these indicators change colour over a range of pH values that includes the pH of NaCl aq the salt produced in the neutralisation reaction. HCl NaOH NaCl H2O During the course of the titration the titrant NaOH is added slowly to the unknown solution.
The point at which exactly enough titrant NaOH has been added to react with all of the analyte HCl is called the equivalence point. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. N10 HCl using methyl orange would not be appropriate here as the color change occurs at 7.
Ammonia solution is a weak base while HCl is a strong acid so methyl orange is suitable indicator. In contrast methyl red begins to change from red to yellow around pH 5 which is near the midpoint of. Just as with the HCl titration the phenolphthalein indicator will turn pink when about 50 mL of N a O H has been added to the acetic acid solution.
From the practical the conclusion made is that 124 ml of NaOH were needed to neutralize and reach the equivalence point of the acidic 150 cm3 HCl. Dont hateI will be. Methyl orange shows pink colour in acidic medium and yellow colour in basic medium.
If you add phenolphthalein indicator to this solution it will have a pink colour. Procedure for titrating a Strong Acid 1. Through the usage of stoichiometry and molarity laws we were able to determine the concentration of the solution which was 009524M.
8 rows NaOH and HCl Titration Curves Selecting Indicators. NaOH is a strong alkali and HCl acid. N NaOH 0013 x 0012 156 x 10-4.
Na2CO3 aq HCl aq NaHCO3 aq NaCl aq Na2CO3 is a basic salt and its solution will have a high pH. Takes on average 119 mL of HCl colorless below pH 82 is complete when the turns. Place 25 mL via volumetric pipet of the HCl solution in a clean labeled 100 mL beaker and add a stirring bar and 1-2 drops of phenolphthalein2.
Phenylphthalene is an OK indicator to use for a titration of NaOH and HCl but its certainly not the best. At this point we have added 4667 ml NaOH. In titration of naoh against hcl 2612 ml of 04624.
Obtain 75 mL of a 0100 M NaOH solution in a clean labeled beaker record exact molarity. In the Titration of a strong base versus a weak acid such as acetic acid PHENOLPHTHALEIN is used as the indicator. Fill a clean 50 mL buret with the NaOH solution take initial reading to 01 mL 4.
A pH indicator shows the equivalence point the point at which the equivalent number of moles of a base have been added to an acid. As it is added the HCl is slowly reacted away. C nV 00001560015 00104 mol dm-3.
Not helpful in this case. A titrant is a solution that is taken inside a burette during titration. Initially starting at a pH of 125 the NaOH was titrated until 4762 mL of HCl was added in which the pH was neutralized at 7.
Then we add NaOH slowly until the indicator color changes. Calculate the precise concentration of the HCl. This is because the pH range of Methyl orange and Phenolphthalein.
In the Titration of a weak base versus a strong acid METHYL ORANGE is used as the indicator. None of the above. When the titration is carried out HCl is aded until the solution turns colorless.
Indicator to use for a titration of NaOH are HCl are used same volumes of NaOH HCl ratio. In the Titration of a strong base versus a weak acid such as acetic acid PHENOLPHTHALEIN is used as the indicator. The titration is complete when the pH reaches 70.
In the given question both NaOH and HCl are strong base and strong acid respectively and so either PHENOLPHTHALEIN or Methyl orange can be used as indicators. H C l is a strong acid and N aOH is a strong base. Find the pH of a mixture of NH3 and HClLots of you guys are messaging me panicking I NEED TITRATION HELPSo heres a rough cut.
HClaqNaOHaq H2OlNaClaq HCl aq NaOH aq H 2 O l NaCl aq Neutralization is the basis of titration. In the Titration of a weak base versus a strong acid METHYL ORANGE is used as the indicator. C NaOH 0013 mol dm3.
For example hydrochloric acid and sodium hydroxide form sodium chloride and water. In the given question both NaOH and HCl are strong base and strong acid respectively and so either PHENOLPHTHALEIN or Methyl orange can be used as indicators.

Titration Chemistry For Non Majors
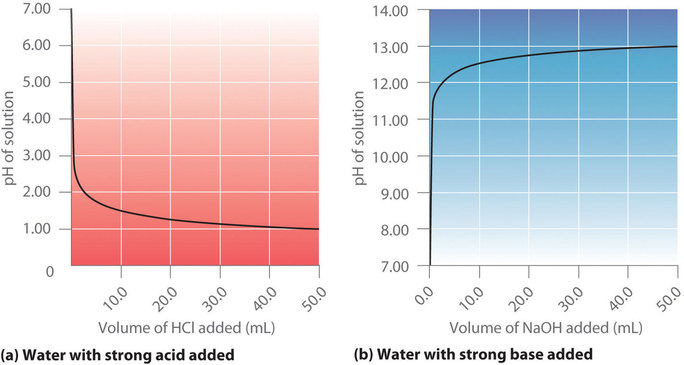
17 4 Neutralization Reactions And Titration Curves Chemistry Libretexts

Strong Acid Strong Base Titrations

14 7 Acid Base Titrations Chemistry Libretexts

9 2 Acid Base Titrations Chemistry Libretexts

Strong Acid Strong Base Titrations

Ph Scale Ph Phscale Mybenznote Baking Soda Naoh Hcl Soap Brocolli Vinegar Tomato Apple Banana Milk Chemical Peel Organic Skin Care Alkaline Diet
Lesson Worksheet Acid Base Titrations Nagwa
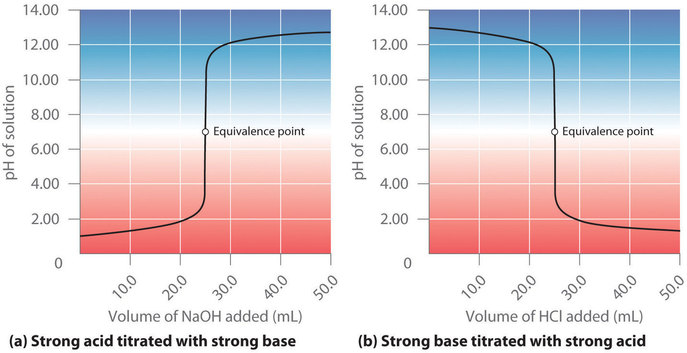
17 4 Neutralization Reactions And Titration Curves Chemistry Libretexts

Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education
Would Phenolphthalein Be The Best Indicator For Naoh And Hcl Titration Quora

9 2 Acid Base Titrations Chemistry Libretexts

9 2 Acid Base Titrations Chemistry Libretexts

Prepare 100 Cm3 Of 0 1m Naoh Solution From 1m Naoh Youtube Chemistry Lecture Solutions Chemistry





Post a Comment for "Best Indicator For Hcl And Naoh Titration"